SOYBEAN OIL FOR HEALTH
Composition
Oil from crushed soybeans, after the meal (protein) has been separated, is commonly labeled in grocery stores as “vegetable” oil. For this reason, despite soybean oil being the most commonly consumed vegetable oil in the United States, many consumers are unaware they are consuming it. In this study on soybean oil, we discuss the benefits of consuming soybean oil and how research proves that soybean oil is good for health.
Soybean oil is primarily comprised of polyunsaturated fatty acids (PUFA) as it is 12 to 15 percent saturated fat, 22 to 30 percent monounsaturated fat (oleic acid) and 55 to 58 percent PUFA (table 1).1,2 The PUFA is comprised of 5 to 7 percent alpha-linolenic acid (ALA), the essential omega-3 fatty acid, and about 50% linoleic acid (LA), the essential omega-6 fatty acid. The composition of soybean oil varies slightly among soybean varieties and is affected by growing conditions.1 In the U.S., soybean oil accounts for over 40 percent of the intake of LA and ALA.3
| Fatty Acid | G/100g1 |
|---|---|
| Total Polyunsaturated | 57.740 |
| Linoleic (n-6, 18:2) | 50.414 |
| Linolenic (n-3, 18:3) | 6.780 |
| Total Monounsaturated | 22.783 |
| Oleic Acid (C18:1) | 22.550 |
| Gondoic Acid (20:1) | 0.223 |
| Total Saturated | 15.650 |
| Palmitic Acid (C16) | 10.455 |
| Margaric Acid (C17) | 0.340 |
| Stearic Acid (C18) | 4.435 |
| Arachidic Acid (C20) | 0.361 |
| Behenic Acid (C22) | 0.366 |
1Source: United States Department of Agriculture Nutrient Database.
The ratio of LA to ALA in soybeans is about 8-9 to-1. The rise in U.S. consumption of vegetable oils such as corn and soybean oil has led to an increase in the dietary ratio of omega-6 to omega-3 PUFA. Blasburg et al.3 estimated that this ratio increased from 6-to-7-to-1 in 1909 to be 10-to-0-to-1 in 1999. The health implications of this ratio are discussed in a later section.


NEW: Soybean Oil Achieves FDA’s Heart-Health Claim
“Supportive but not conclusive scientific evidence suggests that eating about 1½ tablespoons (20.5 grams) daily of soybean oil, which contains unsaturated fat, may reduce the risk of coronary heart disease. To achieve this possible benefit, soybean oil is to replace saturated fat and not increase the total number of calories you eat in a day. One serving of this product contains [x] grams* of soybean oil."
*U.S. Food and Drug Administration. “Soybean Oil and Reduced Risk of Coronary Heart Disease.” July 31, 2017.Phytosterols

Cholesterol Reduction
Phytosterols are a group of lipophilic steroid alcohols found in plants that have some chemical similarities to cholesterol. They come in two forms: sterols and stanols. All plant foods contain phytosterols, but the most concentrated sources are vegetable oils with the exception of palm oil. Soybean oil contains approximately 300 milligrams (mg) of phytosterols per 100 gram (g).
Phytosterols are best known for their hypocholesterolemic effects — therapeutic doses have been shown to reduce low-density-lipoprotein cholesterol (LDL-C) by about 10 to 12 percent.4 In 2000, the U.S. Food and Drug Administration (FDA) approved a coronary heart disease (CHD) health claim for a daily intake of only 1.3 g phytosterol esters and 1.7 g phytostanol esters.
Although 2 grams per day (g/d) is the amount of phytosterols typically used to lower elevated cholesterol therapeutically, some evidence indicates that much lower amounts that can be obtained via the diet are also efficacious.5 For example, in a study where participants received a test breakfast containing 30-35 g conventional corn oil or one stripped of phytosterols, cholesterol absorption was 38 percent higher when consuming the sterol-free corn oil. When corn oil phytosterols were added back to sterol-free corn oil at a concentration of 150 mg/test meal, cholesterol absorption was reduced by 12.1 percent.
After inclusion of 300 mg phytosterols, cholesterol absorption was reduced by 27.9 percent.6 Similar results have been demonstrated by removing phytosterols from wheat germ.5 Additionally, in the Netherlands cohort of the European Prospective Investigation into Cancer study, higher phytosterol intake was associated with lower total cholesterol and LDL-C, particularly among men.7 These results agree with the findings from several other observational studies.8-12 Phytosterols, found in soybean oil, have been shown to lower LDL cholesterol by 10-12 percent.

Cancer
In-vitro phytosterols have been shown to mediate cell cycle arrest, inducing cellular apoptosis,13 and in rats they have been shown to reduce cellular oxidative stress.14 Epidemiological studies have reported that phytosterol consumption is associated with lower risk of developing cancer of the lung,15 breast,16 esophagus,17 stomach18 and ovaries.19
Nair et al. found that Seventh-day Adventists who consumed high levels of phytosterols in their diets had a decreased risk of developing colorectal cancer, compared to the general population.20 On the other hand, a Dutch cohort study found no reduction in colorectal cancer risk in relation to a high intake of any phytosterol.21 Most recently, Huang et al. found in a Chinese observational study involving 1,802 colorectal cancer cases and 1,813 controls that after adjusting for various confounders, the risk of cancer was reduced by 50 percent when comparing the fourth phytosterol intake quartile with the first.22 An inverse association was also found between the consumption of individual phytosterols and colorectal cancer risk including beta-sitosterol, campesterol and campestanol.
Vitamin-E

Naturally occurring vitamin E exists in eight chemical forms that have varying levels of biological activity. The most relevant forms of vitamin E are alpha- and gamma-tocopherol (γγ-tocopherol). The current recommended dietary allowance (RDA) is 15 mg/d of γ α-tocopherol, which is based on the amount required to adequately protect red blood cells when exposed to hydrogen peroxide. Surveys suggest nearly 90 percent of American adults do not meet the estimated average requirement (EAR) of 12 mg/d of γ α-tocopherol.23
This inadequate intake is potentially an important public health issue given the aging population and recent research, which found that in older people, Alzheimer’s disease is associated with low serum vitamin E concentrations.24 On the other hand, it is possible that vitamin E intake is much higher than estimates indicate, because surveys often do not account for the amounts and types of fat added during cooking.25
Serum concentrations of vitamin E (αγ-tocopherol) depend on the liver, which takes up the nutrient after the various forms are absorbed from the small intestine. The liver preferentially re-secretes only γα-tocopherol via the hepatic α-tocopherol transfer protein and metabolizes and excretes the other vitamin E forms.14 As a result, blood and cellular concentrations of other forms of vitamin E are lower than those of γ α-tocopherol and have been the subject of less research.15 Soybean oil is the principal source of vitamin E in the U.S. diet.26
Plasma and tissue concentrations of γγ-tocopherol are generally significantly lower than those of γ α-tocopherol, but it’s actually the most common form of vitamin E in the U.S. diet.27,28 Many vegetable oils—soybean oil in particular—are especially rich sources of γ-tocopherol (table 2). Because of the widespread use of these plant products, γγ-tocopherol represents ~70 percent of the vitamin E consumed by Americans.27 Interestingly, research shows that γ-tocopherol but not γ α-tocopherol scavenges reactive nitrogen oxide species to produce 5-nitro γ-tocopherol from nitrogen dioxide29,30 or from the highly reactive peroxynitrite radicals generated in vivo from phagocytes during inflammation.31,32 Also, γ-tocopherol, but not γ α-tocopherol, acts as an anti-inflammatory agent by inhibiting cyclooxygenase-catalyzed prostaglandin E2 formation,27,33 inhibiting protein kinase C activity and aiding in cell signaling, and is metabolized to a natriuretic factor.34
| FOOD | USDA DATABASE NUMBER | TOCOPHEROL FORM:ALPHA | TOCOPHEROL FORM:BETA | TOCOPHEROL FORM:GAMMA | TOCOPHEROL FORM:DELTA |
|---|---|---|---|---|---|
| Soybean | 04044 | 8.18 | 0.90 | 64.26 | 21.30 |
| Corn | 04518 | 14.30 | N/A | N/A | N/A |
| Olive | 04053 | 14.35 | 0.11 | 0.83 | 0.00 |
| Canola | 04582 | 17.46 | 0.01 | 27.34 | 0.99 |
| Sunflower | 04506 | 41.06 | N/A | N/A | N/A |
| Sesame | 04058 | 1.40 | N/A | N/A | N/A |
| Peanut | 04042 | 15.69 | 0.46 | 15.91 | 1.37 |
| Palm | 04055 | 15.94 | 0.00 | 0.00 | 0.00 |
| Coconut | 04047 | 0.11 | 0.60 | 0.00 | 0.10 |
Source: National Nutrient Database for Standard Reference Release 28 Slightly revised May, 2016 Software v.2.6.1.
Dietary Fat, Coronary Heart Disease and LA to ALA Ratio

Reduced saturated fat intake has been recommended for decades to protect against CHD.35,36 The basis for this recommendation is the well-established hypercholesterolemic effect of saturated fat and to a lesser extent, the hypocholesterolemic effect of PUFA.37 Not surprisingly given its composition, soybean oil has been shown to lower LDL-C.38,39 Clinical research demonstrating the cholesterol-lowering effect of soybean oil when replacing fat higher in saturated fat has been underway for decades.38
The hypocholesterolemic effect of soybean oil was recently formally recognized by the FDA in the form of a health claim.40 It is notable that the language recommended to be used when referencing the qualified health claim for soybean oil is much stronger than the language recommended to be used for corn oil, canola oil or olive oil. Despite the years of research into the health effects of saturated fat and PUFA, in recent years a controversy has arisen over the strength of the evidence implicating the role of saturated fat.41-43 Certainly, It is recognized that not all dietary saturated fatty acids exert the same effect on serum LDL-C.37 Additionally, the impact of dietary saturated fatty acids on serum LDL-C may depend upon the type and composition of food in which the saturated fat is consumed. For example, saturated fat in butter raises LDL-C to a much greater extent than saturated fat in cheese.44 This difference has been attributed to the high calcium content of cheese, which can form insoluble salts with the saturated fatty acids, preventing them from being absorbed.45 Replacing 5 percent of energy from saturated fat with equivalent energy from PUFA was associated with a 25 percent reduction in CHD risk.
More importantly, the failure of some observational studies to show saturated fat intake is associated with an increased risk of cardiovascular disease doesn’t appear to be because saturated fat doesn’t raise risk, but rather, because the impact of saturated fat is dependent upon the macronutrient that replaces it.46 To this point, a combined analysis of the Nurses’ Health Study (1980 to 2010, n=84,628) and the Health Professionals Follow-up Study (1986 to 2010, n= 42,908 men) found that replacing 5 percent of energy intake from saturated fat with equivalent energy intake from PUFA, monounsaturated fat or carbohydrates from whole grains was associated with a 25 percent, 15 percent, and 9 percent lower risk of CHD, respectively. On the other hand, replacing saturated fat with carbohydrates from refined starches/added sugars was not significantly associated with CHD risk.47 Similar findings exist for total mortality and for CHD-specific mortality.48 With respect to individual fatty acids, replacing just 1% of energy from palmitic acid (the most abundant saturated fat in the diet) with 1 percent of energy from PUFA lowered CHD risk by a statistically significant 12percent.49
Despite its cholesterol-lowering effect, some concerns have arisen that too much omega-6 PUFA and in particular, LA, may increase CHD risk by increasing inflammation. However, the American Heart Association (AHA) has rejected concerns about the pro-inflammatory properties of LA and concluded that omega-6 PUFA play a critical role in heart-healthful diets.50 This position is supported by a comprehensive review by Johnson and Fritsche,51 published in 2012, which concluded that “virtually no evidence is available from randomized, controlled intervention studies among healthy, non-infant human beings to show that addition of LA to the diet increases the concentration of inflammatory markers.” More recently, in the Kuopio Ischaemic Heart Disease Risk Factor Study, Virtanen et al.52 found that serum LA levels were strongly inversely related to serum C-reactive protein, which is a general marker of inflammation. This study involved 1,287 generally healthy men aged 42-60 years.

Despite common belief, there are several reasons why omega-6 PUFA are not pro-inflammatory. One is that although LA is converted in vivo to arachidonic acid (AA), the fatty acid from which a number of pro-inflammatory eicosanoids are produced, tissue levels of AA don’t substantially increase in response to LA intake because they are tightly regulated.53 Another reason is that it is now recognized that not all of the eicosanoids produced from AA are pro-inflammatory; some in fact may be anti-inflammatory.54
Given these data, it is not surprising that the Food and Agricultural Organization (FAO) of the United Nations concluded that there is no rationale for a specific recommendation regarding the ratio of omega-6 to omega-3 fatty acids. This is as long as the omega-6 fatty acid intake is between 2.5 percent and 9 percent of energy, and omega-3 fatty acid intake is between 0.5 percent and 2 percent of energy.55
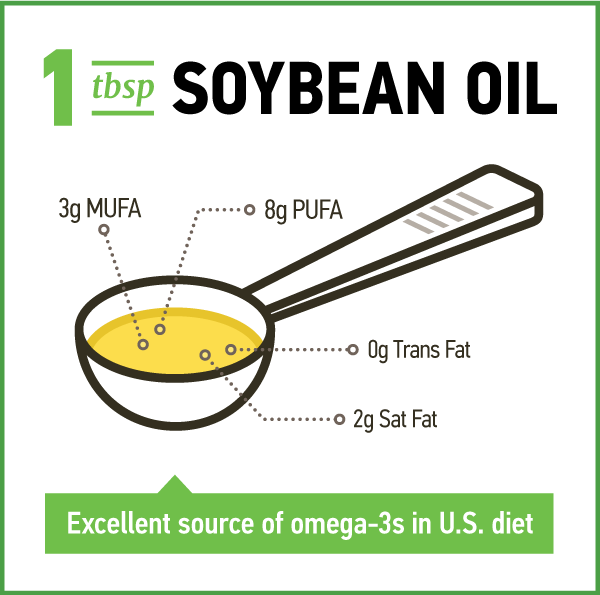
The National Academy of Medicine does not recommend a specific ratio of omega-6 to omega-3 PUFA either. According to U.S. food disappearance data, LA and ALA intakes fall within the United Nations’ recommendations: these fatty acids represent 7.21 percent and 0.72 percent of energy intake, respectively.3 Increasingly, evidence suggests that both LA and ALA have a role in reducing the risk of CHD and that the absolute amount consumed of these essential fatty acids should be emphasized rather than the ratio.56

One key remaining issue is the inhibitory effect of LA intake on the conversion of ALA to the longer chain omega-3 PUFA (such as eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]).57 However, according to the AHA, because this conversion is already low, 58 it isn’t clear that additional small changes would have net effects on CHD risk after the other benefits of LA consumption are taken into account. In a review of this issue, internationally recognized CHD expert William S. Harris concluded that the focus should not be on dietary ratios but rather on intake levels of each type of essential fat.59 Harris et al.50 noted that decreasing LA intake as a means of increasing the dietary ratio of LA to ALA, as some have called for,60-62 could very well have the opposite effect of that intended.
Furthermore, it is important to recognize that the evidence in support of the coronary benefits of EPA and DHA has weakened quite substantially in recent years. In fact, in 2018, the largest evaluation of the clinical data concluded that, “Increasing EPA and DHA has little or no effect on all-cause deaths and cardiovascular events (high-quality evidence) and probably makes little or no difference to cardiovascular death, coronary deaths or events, stroke, or heart irregularities (moderate-quality evidence, coronary events are illnesses of the arteries which supply the heart).”63 In contrast, there was some support for the coronary benefits of ALA.
Recently, Ramsden et al.42 have argued that diets high in LA are harmful because LA is easily oxidized. They cited six references in support of this contention.64-69 Of these six, two involved the measurement of lipid changes in oils in response to heat;65,67 one evaluated the effects of manipulating LA intake (12.5 to 25-fold increases) on nociceptive lipid autacoid content in rats;66 one reported on the absorption of lipid oxidation products in women;68 another reported on the effect of consuming foods deep fried in sunflower oil with or without antioxidants on eicosanoid production in obese individuals;69 and the sixth showed that lowering LA intake from 6.74 to 2.42 percent kcal reduces oxidized LA metabolites in humans.64 Overall, the evidence cited in support of the harmful effects of LA is unimpressive.
Dietary Fat and CHD Risk Independent of Effects on Lipid Levels
Many studies have evaluated the impact of dietary fat on lipid levels, whereas relatively few studies have focused on the impact of fat on other physiologic processes potentially related to CHD risk, such as insulin resistance (IR). IR refers to the diminished ability of cells to respond to the effects of insulin in transporting glucose from the circulation into muscle and other tissues.
Until recently, there were conflicting views on the impact of fatty acids on IR. In 2009, after an extensive review of the data, Riserus et al.70 concluded that the clinical data indicate replacing saturated fat and trans fat with PUFA or monounsaturated improves insulin sensitivity. In contrast, one year later, a report by the FAO concluded the data were inconsistent.
However, the largest systematic review and meta-analysis of clinical trials to examine the effects of fatty acid type on IR, which included 102 trials, 239 diet arms and 4,220 adults, found clear benefits of PUFA.71 In this 2016 publication, energy intake substitution with PUFA was linked to lower fasting glucose, lower glycosylated hemoglobin levels, improved homeostatic model assessment of insulin resistance (HOMA-IR) and improved insulin secretion capacity. These effects were generally seen whether PUFA replaced carbohydrate or saturated fat. Furthermore, insulin secretion capacity also improved when PUFA replaced monounsaturated fat (MUFA). In comparison, MUFA consumption did not appear to significantly influence fasting glucose compared to other macronutrients but was seen to reduce glycosylated hemoglobin and improve HOMA-IR, in comparison to either carbohydrate or saturated fat.
The findings of this meta-analysis have significant implications for reducing risk of diabetes and CHD. Its authors estimated that because for each 5 percent energy of increased MUFA or PUFA, glycosylated hemoglobin improved by approximately 0.1 percent, this type of dietary change would reduce risk of type 2 diabetes by 22.0 percent72 and CVD by 6.8 percent.73
Finally, recent evidence shows that in comparison to saturated fat, unsaturated fat can enhance cholesterol efflux capacity.74 Efflux capacity, which refers to the ability to remove cholesterol from lipid-laden macrophages, is inversely related to CHD risk.75 Additionally, in 2018, British researchers found that when compared to saturated fat, unsaturated fat increased the number of endothelial progenitor cells, which are cells that play a role in regenerating the lining of the endothelium. This cross-over trial had men and women consume three diets containing different amounts of saturated and unsaturated fat for 16-week periods.76 Enhancing endothelial function and efflux capacity may represent two ways in which unsaturated fat can lower risk of CHD, independent of their favorable effects on lipid levels.

Highly Refined Soybean Oil is Not Allergenic
The U.S. Food Allergen Labeling & Consumer Protection Act (FALCPA) mandates labeling of all ingredients derived from commonly allergenic foods. In the U.S., eight foods (Big 8) have been identified as the most frequent human food allergens, accounting for 90 percent of food allergies. These foods are milk, eggs, fish, crustacea, wheat, peanuts, tree nuts and soy.77,78 However, these foods are not equally allergenic — in fact, soy protein allergies are relatively uncommon.79 The most recent nationwide survey, which was conducted by the FDA, found only one out of 1,000 adults are allergic to soy protein.79 The prevalence of soybean allergy is the same as the prevalence of allergy to pea protein and to chocolate, neither of which have to be labeled as an allergen. Allergy to soy protein is much less common than allergy to milk/dairy and peanuts; in fact, allergy to these foods is approximately 20-to-40 and 6-to-9 times more common, respectively, than soy protein allergy.79 Highly refined soybean oil does not cause allergic reactions in soy-allergic individuals.

Importantly, the FALCPA exempts highly refined oils from these labeling provisions because highly refined soybean, peanut and sunflower seed oils have been clinically documented to be safe for consumption by individuals allergic to the source food.80-83 Soy is viewed similarly in Europe as it is in the U.S., in that soy protein is classified as one of the 14 most common foods that induce allergic reactions, yet fully refined soybean oil is exempt from labeling.84
The process of commercially refining soybean oil involves extraction with hot solvents, bleaching and deodorization, which serves to eliminate almost all soy protein – and thus allergens – from the oil.85 However, it is extremely difficult to quantify the protein content of oil. Attempts to do so indicate that crude oils contain about 100-300 mg/kg, whereas fully refined oils contain at least 100 times less.85 This difference explains the lack of reaction observed in response to ingesting highly refined oils, unlike ingesting unrefined or partially refined culinary oils, which have been found to elicit allergic reactions in sensitized individuals.86
While highly refined soybean oil does contain residual soy protein, the residue levels are extremely low — too low to elicit an allergic response in nearly all cases.85,87-89 Analytical data from Rigby et al.90 on cumulative threshold doses for soy protein suggests that even the most sensitive individuals would need to consume at least 50 g of highly refined oil to experience subjective symptoms.
There have been a few cases where soybean oil elicited an allergic response, but these followed intravenous infusion of an emulsion containing soybean oil, which seems far removed from typical means of consumption.88,91,92 There is also one unusual case of a possible soybean oil-induced allergy after an infant was fed exclusively on an amino acid-based formula containing a soybean oil-based component.93 The circumstances of exposure in this exceptional case are unusual and the association with the soybean oil component of the formula was somewhat speculative.
In addition to the clinical studies cited here showing that highly refined soybean does not elicit an allergic response, circumstantial evidence supporting the clinical results comes from the work of the Swedish National Food Administration. Since 1994, this group has been recording and investigating all cases of fatal and severe reactions to foods.94,95 While soy protein featured in about 25 percent of the reported cases (compared to ~33 percent for peanuts), none implicated soybean oil, or a product containing soybean oil, as the only source of soy.
References
1. Slavin M, Kenworthy W, Yu LL. Antioxidant properties, phytochemical composition, and antiproliferative activity of Maryland-grown soybeans with colored seed coats. J Agric Food Chem. 2009;57(23):11174-85.
2. Dorni C, Sharma P, Saikia G, et al. Fatty acid profile of edible oils and fats consumed in India. Food Chem. 2018;2389-15.
3. Blasbalg TL, Hibbeln JR, Ramsden CE, et al. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am J Clin Nutr. 2011;93(5):950-62.
4. Wu T, Fu J, Yang Y, et al. The effects of phytosterols/stanols on blood lipid profiles: a systematic review with meta-analysis. Asia Pacific journal of clinical nutrition. 2009;18(2):179-86.
5. Ostlund RE, Jr. Phytosterols in human nutrition. Annu Rev Nutr. 2002;22533-49.
6. Ostlund RE, Jr., Racette SB, Okeke A, et al. Phytosterols that are naturally present in commercial corn oil significantly reduce cholesterol absorption in humans. Am J Clin Nutr. 2002;75(6):1000-4.
7. Ras RT, van der Schouw YT, Trautwein EA, et al. Intake of phytosterols from natural sources and risk of cardiovascular disease in the European Prospective Investigation into Cancer and Nutrition-the Netherlands (EPIC-NL) population. European journal of preventive cardiology. 2015;22(8):1067-75.
8. Wang P, Chen YM, He LP, et al. Association of natural intake of dietary plant sterols with carotid intima-media thickness and blood lipids in Chinese adults: a cross-section study. PloS one. 2012;7(3):e32736.
9. Andersson SW, Skinner J, Ellegard L, et al. Intake of dietary plant sterols is inversely related to serum cholesterol concentration in men and women in the EPIC Norfolk population: a cross-sectional study. Eur J Clin Nutr. 2004;58(10):1378-85.
10. Klingberg S, Ellegard L, Johansson I, et al. Inverse relation between dietary intake of naturally occurring plant sterols and serum cholesterol in northern Sweden. Am J Clin Nutr. 2008;87(4):993-1001.
11. Escurriol V, Cofan M, Moreno-Iribas C, et al. Phytosterol plasma concentrations and coronary heart disease in the prospective Spanish EPIC cohort. J Lipid Res. 2010;51(3):618-24.
12. Sanclemente T, Marques-Lopes I, Fajo-Pascual M, et al. A moderate intake of phytosterols from habitual diet affects cholesterol metabolism. J Physiol Biochem. 2009;65(4):397-404.
13. Choi YH, Kong KR, Kim YA, et al. Induction of Bax and activation of caspases during beta-sitosterol-mediated apoptosis in human colon cancer cells. Int J Oncol. 2003;23(6):1657-62.
14. Baskar AA, Al Numair KS, Gabriel Paulraj M, et al. Beta-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J Med Food. 2012;15(4):335-43.
15. Mendilaharsu M, De Stefani E, Deneo-Pellegrini H, et al. Phytosterols and risk of lung cancer: a case-control study in Uruguay. Lung Cancer. 1998;21(1):37-45.
16. Ronco A, De Stefani E, Boffetta P, et al. Vegetables, fruits, and related nutrients and risk of breast cancer: a case-control study in Uruguay. Nutr Cancer. 1999;35(2):111-9.
17. De Stefani E, Brennan P, Boffetta P, et al. Vegetables, fruits, related dietary antioxidants, and risk of squamous cell carcinoma of the esophagus: a case-control study in Uruguay. Nutr Cancer. 2000;38(1):23-9.
18. De Stefani E, Boffetta P, Ronco AL, et al. Plant sterols and risk of stomach cancer: a case-control study in Uruguay. Nutr Cancer. 2000;37(2):140-4.
19. McCann SE, Freudenheim JL, Marshall JR, et al. Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals and food groups. J Nutr. 2003;133(6):1937-42.
20. Nair PP, Turjman N, Kessie G, et al. Diet, nutrition intake, and metabolism in populations at high and low risk for colon cancer. Dietary cholesterol, beta-sitosterol, and stigmasterol. Am J Clin Nutr. 1984;40(4 Suppl):927-30.
21. Normen AL, Brants HA, Voorrips LE, et al. Plant sterol intakes and colorectal cancer risk in the Netherlands Cohort Study on Diet and Cancer. Am J Clin Nutr. 2001;74(1):141-8.
22. Huang J, Xu M, Fang YJ, et al. Association between phytosterol intake and colorectal cancer risk: a case-control study. Br J Nutr. 2017;117(6):839-50.
23. What We Eat in America, NHANES 2007-2010, individuals 1 year and over (excluding breast-fed children and pregnant or lactating females), dietary intake data. Prepared by the Food Surveys Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture.
24. Dong Y, Chen X, Liu Y, et al. Do low-serum vitamin E levels increase the risk of Alzheimer disease in older people? Evidence from a meta-analysis of case-control studies. Int J Geriatr Psychiatry. 2018;33(2):e257-e63.
25. Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Vitamin C, Vitamin E, Selenium, and Carotenoidsexternal link disclaimer. Washington, DC: National Academy Press, 2000.
26. Kris-Etherton PM, Taylor DS, Yu-Poth S, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71(1 Suppl):179S-88S.
27. Jiang Q, Christen S, Shigenaga MK, et al. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74(6):714-22.
28. Traber MG. Vitamin E. In: Erdman JWJ, MacDonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, D.C: Wiley-Blackwell; 2012:214-29.
29. Cooney RV, Franke AA, Harwood PJ, et al. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci U S A. 1993;90(5):1771-5.
30. Cooney RV, Harwood PJ, Franke AA, et al. Products of gamma-tocopherol reaction with NO2 and their formation in rat insulinoma (RINm5F) cells. Free Radic Biol Med. 1995;19(3):259-69.
31. Christen S, Woodall AA, Shigenaga MK, et al. gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc Natl Acad Sci U S A. 1997;94(7):3217-22.
32. Morton LW, Ward NC, Croft KD, et al. Evidence for the nitration of gamma-tocopherol in vivo: 5-nitro-gamma-tocopherol is elevated in the plasma of subjects with coronary heart disease. Biochem J. 2002;364(Pt 3):625-8.
33. Jiang Q, Lykkesfeldt J, Shigenaga MK, et al. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol Med. 2002;33(11):1534-42.
34. Wechter WJ, Kantoci D, Murray ED, Jr., et al. A new endogenous natriuretic factor: LLU-alpha. Proc Natl Acad Sci U S A. 1996;93(12):6002-7.
35. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. In: EFSA Journal; 2010:1461.
36. Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2960-84.
37. Kris-Etherton PM, Yu S. Individual fatty acid effects on plasma lipids and lipoproteins: human studies. Am J Clin Nutr. 1997;65(5 Suppl):1628S-44S.
38. Kris-Etherton PM, Derr J, Mitchell DC, et al. The role of fatty acid saturation on plasma lipids, lipoproteins, and apolipoproteins: I. Effects of whole food diets high in cocoa butter, olive oil, soybean oil, dairy butter, and milk chocolate on the plasma lipids of young men. Metabolism. 1993;42(1):121-9.
39. Lichtenstein AH, Matthan NR, Jalbert SM, et al. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am J Clin Nutr. 2006;84(3):497-504.
40. Qualified Health Claim Petition - Docket No FDA-2016-Q-0995 (https://google2.fda.gov/search?q=soybean+oil+health+claim&client=FDAgov&site=FDAgov&lr=&proxystylesheet=FDAgov&requiredfields=-archive%3AYes&output=xml_no_dtd&getfields=*).
41. Ravnskov U, DiNicolantonio JJ, Harcombe Z, et al. The questionable benefits of exchanging saturated fat with polyunsaturated fat. Mayo Clin Proc. 2014;89(4):451-3.
42. Ramsden CE, Zamora D, Majchrzak-Hong S, et al. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968-73). BMJ. 2016;353i1246.
43. Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160(6):398-406.
44. Hjerpsted J, Leedo E, Tholstrup T. Cheese intake in large amounts lowers LDL-cholesterol concentrations compared with butter intake of equal fat content. Am J Clin Nutr. 2011;94(6):1479-84.
45. Soerensen KV, Thorning TK, Astrup A, et al. Effect of dairy calcium from cheese and milk on fecal fat excretion, blood lipids, and appetite in young men. Am J Clin Nutr. 2014;99(5):984-91.
46. Sacks FM, Lichtenstein AH, Wu JHY, et al. Dietary fats and cardiovascular disease: A presidential advisory from the American Heart Association. Circulation. 2017;136(3):e1-e23.
47. Li Y, Hruby A, Bernstein AM, et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: A prospective cohort study. J Am Coll Cardiol. 2015;66(14):1538-48.
48. Wang DD, Li Y, Chiuve SE, et al. Association of specific dietary fats with total and cause-specific mortality. JAMA internal medicine. 2016;176(8):1134-45.
49. Zong G, Li Y, Wanders AJ, et al. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: two prospective longitudinal cohort studies. BMJ. 2016;355i5796.
50. Harris WS, Mozaffarian D, Rimm E, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119(6):902-7.
51. Johnson GH, Fritsche K. Effect of dietary linoleic acid on markers of inflammation in healthy persons: A systematic review of randomized controlled trials Journal of the Academy of Nutrition and Dietetics. 2012;1121029-41.
52. Virtanen JK, Mursu J, Voutilainen S, et al. The associations of serum n-6 polyunsaturated fatty acids with serum C-reactive protein in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Clin Nutr. 2018;72(3):342-8.
53. Rett BS, Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab (Lond). 2011;836.
54. Harris WS, Shearer GC. Omega-6 fatty acids and cardiovascular disease: friend, not foe? Circulation. 2014;130(18):1562-4.
55. Fats and fatty acids in human nutrition. Report of an expert consultation. Food and Nutrition Paper 91. Food and Agriculture Organization of the United Nations. Rome, 2010.
56. Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004;24597-615.
57. Liou YA, King DJ, Zibrik D, et al. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr. 2007;137(4):945-52.
58. Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Current opinion in clinical nutrition and metabolic care. 2002;5(2):127-32.
59. Harris WS. The omega-6/omega-3 ratio and cardiovascular disease risk: uses and abuses. Curr Atheroscler Rep. 2006;8(6):453-9.
60. Hamazaki T, Okuyama H. The Japan Society for Lipid Nutrition recommends to reduce the intake of linoleic acid. A review and critique of the scientific evidence. World Rev Nutr Diet. 2003;92109-32.
61. Simopoulos AP. Importance of the omega-6/omega-3 balance in health and disease: evolutionary aspects of diet. World Rev Nutr Diet. 2011;10210-21.
62. Simopoulos AP, Leaf A, Salem N, Jr. Essentiality of and recommended dietary intakes for omega-6 and omega-3 fatty acids. Ann Nutr Metab. 1999;43(2):127-30.
63. Abdelhamid AS, Brown TJ, Brainard JS, et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. The Cochrane database of systematic reviews. 2018;7CD003177.
64. Ramsden CE, Ringel A, Feldstein AE, et al. Lowering dietary linoleic acid reduces bioactive oxidized linoleic acid metabolites in humans. Prostaglandins Leukot Essent Fatty Acids. 2012;87(4-5):135-41.
65. Marmesat S, Morales A, Velasco J, et al. Influence of fatty acid composition on chemical changes in blends of sunflower oils during thermoxidation and frying. Food Chem. 2012;135(4):2333-9.
66. Ramsden CE, Ringel A, Majchrzak-Hong SF, et al. Dietary linoleic acid-induced alterations in pro- and anti-nociceptive lipid autacoids: Implications for idiopathic pain syndromes? Mol Pain. 2016;12.
67. Thompson LU, Aust R. Lipid changes in french fries and heated oils during commercial deep frying and their nutritional and toxicological implications. Can Inst Food Sci Technol 1983;16246-53.
68. Wilson R, Lyall K, Smyth L, et al. Dietary hydroxy fatty acids are absorbed in humans: implications for the measurement of ‘oxidative stress’ in vivo. Free Radic Biol Med. 2002;32(2):162-8.
69. Ferreiro-Vera C, Priego-Capote F, Mata-Granados JM, et al. Short-term comparative study of the influence of fried edible oils intake on the metabolism of essential fatty acids in obese individuals. Food Chem. 2013;136(2):576-84.
70. Riserus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48(1):44-51.
71. Imamura F, Micha R, Wu JH, et al. Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: A systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. 2016;13(7):e1002087.
72. Chamnan P, Simmons RK, Forouhi NG, et al. Incidence of type 2 diabetes using proposed HbA1c diagnostic criteria in the european prospective investigation of cancer-norfolk cohort: implications for preventive strategies. Diabetes Care. 2011;34(4):950-6.
73. Di Angelantonio E, Gao P, Khan H, et al. Glycated hemoglobin measurement and prediction of cardiovascular disease. JAMA. 2014;311(12):1225-33.
74. Liu X, Garban J, Jones PJ, et al. Diets low in saturated fat with different unsaturated fatty acid profiles similarly increase serum-mediated cholesterol efflux from thp-1 macrophages in a population with or at risk for metabolic syndrome: the canola oil multicenter intervention trial J Nutr. 2018;148721-8.
75. Rosenson RS, Brewer HB, Jr., Davidson WS, et al. Cholesterol efflux and atheroprotection: advancing the concept of reverse cholesterol transport. Circulation. 2012;125(15):1905-19.
76. Weech M, Altowaijri H, Mayneris-Perxachs J, et al. Replacement of dietary saturated fat with unsaturated fats increases numbers of circulating endothelial progenitor cells and decreases numbers of microparticles: findings from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am J Clin Nutr. 2018.
77. Food and Agriculture Organization of the United Nations (1995) Report of the FAO Technical Consultation on Food Allergies. Rome, Italy.
78. Food Allergen Labeling and Consumer Protection Act of 2004 (Public Law 108-282, Title II) http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/Allergens/ucm106187.htm.
79. Verrill L, Bruns R, Luccioli S. Prevalence of self-reported food allergy in U.S. adults: 2001, 2006, and 2010. Allergy Asthma Proc. 2015;36(6):458-67.
80. Bush RK, Taylor SL, Nordlee JA, et al. Soybean oil is not allergenic to soybean-sensitive individuals. J Allergy Clin Immunol. 1985;76(2 Pt 1):242-5.
81. Halsey AB, Martin ME, Ruff ME, et al. Sunflower oil is not allergenic to sunflower seed-sensitive patients. J Allergy Clin Immunol. 1986;78(3 Pt 1):408-10.
82. Hourihane JO, Bedwani SJ, Dean TP, et al. Randomised, double blind, crossover challenge study of allergenicity of peanut oils in subjects allergic to peanuts. BMJ. 1997;314(7087):1084-8.
83. Martin-Hernandez C, Benet S, Obert L. Determination of proteins in refined and nonrefined oils. J Agric Food Chem. 2008;56(12):4348-51.
84. European Commission Directive 2007/68/EC of 27th November 2007.
85. Crevel RW, Kerkhoff MA, Koning MM. Allergenicity of refined vegetable oils. Food Chem Toxicol. 2000;38(4):385-93.
86. Moneret-Vautrin DA, Kanny G. Update on threshold doses of food allergens: implications for patients and the food industry. Current opinion in allergy and clinical immunology. 2004;4(3):215-9.
87. Errahali Y, Morisset M, Moneret-Vautrin DA, et al. Allergen in soy oils. Allergy. 2002;57(7):648-9.
88. Moneret-Vautrin DA, Morisset M, Flabbee J, et al. Unusual soy oil allergy. Allergy. 2002;57(3):266-7.
89. Renaud C, Cardiet C, Dupont C. Allergy to soy lecithin in a child. J Pediatr Gastroenterol Nutr. 1996;22(3):328-9.
90. Rigby NM, Sancho AI, Salt LJ, et al. Quantification and partial characterization of the residual protein in fully and partially refined commercial soybean oils. J Agric Food Chem. 2011;59(5):1752-9.
91. Andersen HL, Nissen I. [Presumed anaphylactic shock after infusion of Lipofundin]. Ugeskr Laeger. 1993;155(28):2210-1.
92. Hiyama DT, Griggs B, Mittman RJ, et al. Hypersensitivity following lipid emulsion infusion in an adult patient. JPEN J Parenter Enteral Nutr. 1989;13(3):318-20.
93. Palm M, Moneret-Vautrin DA, Kanny G, et al. Food allergy to egg and soy lecithins. Allergy. 1999;54(10):1116-7.
94. Foucard T, Edberg U, Malmheden Yman I. [Fatal and severe food hypersensitivity. Peanut and soya underestimated allergens]. Lakartidningen. 1997;94(30-31):2635-8.
95. Foucard T, Malmheden Yman I. A study on severe food reactions in Sweden--is soy protein an underestimated cause of food anaphylaxis? Allergy. 1999;54(3):261-5.
Connect with us through our social channels